Answer to Solved 11. Carbon absorbs energy at a wavelength of 150 nm, Science; Chemistry; Chemistry questions and answers; 11. Carbon absorbs energy at a wavelength of 150 nm, the total amount of energy emitted by carbon is 1.88×105 J. Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon.
A state-of-the-art review on coir fiber-reinforced biocomposites – RSC Advances (RSC Publishing) DOI:10.1039/D1RA00231G
Question: Carbon absorbs energy at a wavelength of 150 nm. The total amount of energy emitted by a specific carbon sample is 1.98×105 J. Calculate the mass of the carbon sample assuming that each carbon atom emits one photon. Show transcribed image text. There are 3 steps to solve this one.
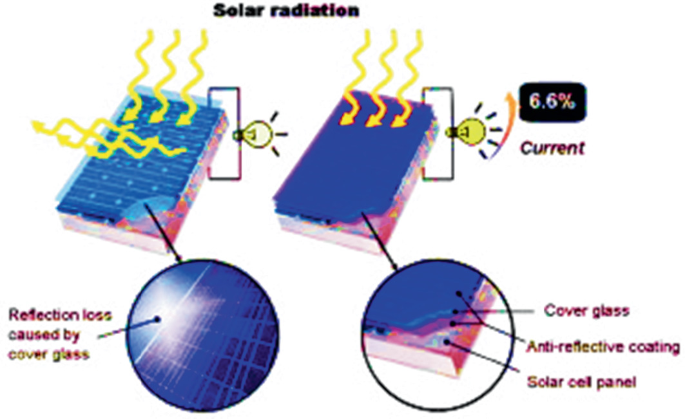
Source Image: link.springer.com
Download Image
Jan 18, 2024To find the energy in joules given the wavelength of a photon: Use Planck’s equation E = h x c / λ and substitute the values of the wavelength (λ), Planck’s constant in joules (h = 6.6261 × 10⁻³⁴ J·s), and speed of light (c = 299792458 m/s). With these units, you’ll get an energy result in joules (J). That’s it!
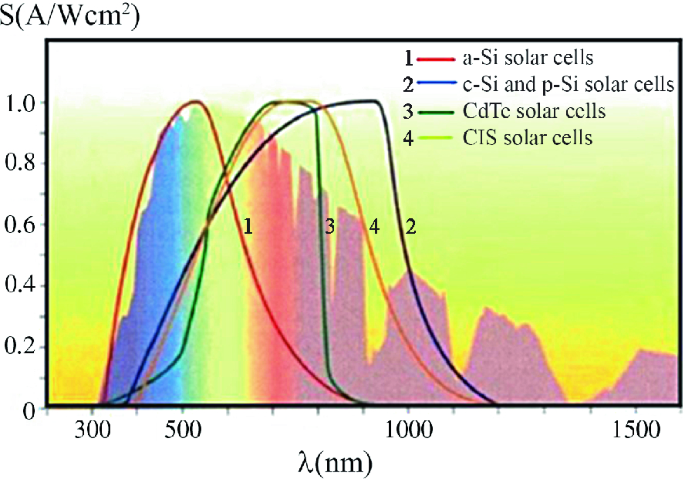
Source Image: link.springer.com
Download Image
A state-of-the-art review on coir fiber-reinforced biocomposites – RSC Advances (RSC Publishing) DOI:10.1039/D1RA00231G Carbon absorbs energy at a wavelength of $150 . \mathrmnm.$ The total amount of energy emitted by a carbon sample is $1.98 \times 10^5 \mathrmJ$ Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon. 02:44.

Source Image: judithcurry.com
Download Image
Carbon Absorbs Energy At A Wavelength Of 150
Carbon absorbs energy at a wavelength of $150 . \mathrmnm.$ The total amount of energy emitted by a carbon sample is $1.98 \times 10^5 \mathrmJ$ Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon. 02:44. VIDEO ANSWER: If a carbon atom emits a wavelength of 100 and 50 nanometers, then it will also emit a wavelength of 100 and 50 nanometers. … Carbon absorbs energy at a wavelength of 150. nm The total amount of energy emitted by a carbon sample is 2.23 x 105 J. Calculate the number of carbon atoms present in the sample, assuming that each atom
TORNADO | Climate Etc.
Find stepby-step Chemistry solutions and your answer to the following textbook question: Carbon absorbs energy at a wavelength of 150. nm. The total amount of energy emitted by a carbon sample is $1.98 \times 10 ^ 5 $ J. Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon.. 한국도시설계학회 urban design Unbearable awareness is
Source Image: panafrican-med-journal.com
Download Image
PDF) Review of Active and Passive Daylighting Technologies for Sustainable Building Find stepby-step Chemistry solutions and your answer to the following textbook question: Carbon absorbs energy at a wavelength of 150. nm. The total amount of energy emitted by a carbon sample is $1.98 \times 10 ^ 5 $ J. Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon..

Source Image: researchgate.net
Download Image
A state-of-the-art review on coir fiber-reinforced biocomposites – RSC Advances (RSC Publishing) DOI:10.1039/D1RA00231G Answer to Solved 11. Carbon absorbs energy at a wavelength of 150 nm, Science; Chemistry; Chemistry questions and answers; 11. Carbon absorbs energy at a wavelength of 150 nm, the total amount of energy emitted by carbon is 1.88×105 J. Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon.

Source Image: pubs.rsc.org
Download Image
A state-of-the-art review on coir fiber-reinforced biocomposites – RSC Advances (RSC Publishing) DOI:10.1039/D1RA00231G Jan 18, 2024To find the energy in joules given the wavelength of a photon: Use Planck’s equation E = h x c / λ and substitute the values of the wavelength (λ), Planck’s constant in joules (h = 6.6261 × 10⁻³⁴ J·s), and speed of light (c = 299792458 m/s). With these units, you’ll get an energy result in joules (J). That’s it!
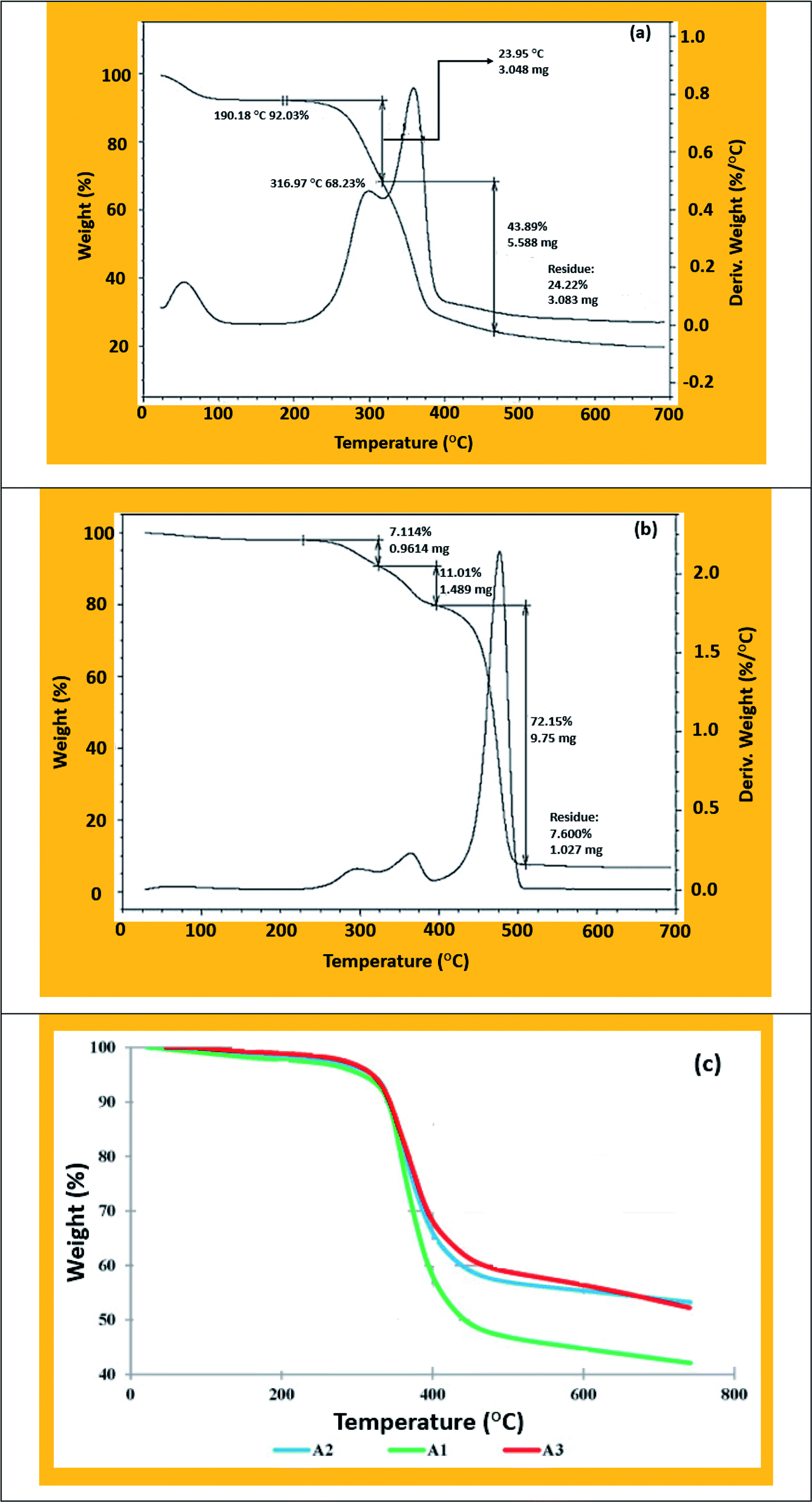
Source Image: pubs.rsc.org
Download Image
ISO Calculate the energy and wavelength of a photon of light with a frequency of 6.165 x 10^14 Hz. Calculate the energy of a photon of wavelength 0.154nm; Calculate the energy of a photon having a wavelength of 5.42×10-6 m. Calculate the energy per photon of a wavelength of 6.0 x 10-10 m. Calculate the energy per photon of a wavelength of 3.0 x 10-3 m.

Source Image: yumpu.com
Download Image
⏩SOLVED:Carbon absorbs energy at a wavelength of 150 . nm . The… | Numerade Carbon absorbs energy at a wavelength of $150 . \mathrmnm.$ The total amount of energy emitted by a carbon sample is $1.98 \times 10^5 \mathrmJ$ Calculate the number of carbon atoms present in the sample, assuming that each atom emits one photon. 02:44.

Source Image: numerade.com
Download Image
5 Optical Mineralogy – Mineralogy VIDEO ANSWER: If a carbon atom emits a wavelength of 100 and 50 nanometers, then it will also emit a wavelength of 100 and 50 nanometers. … Carbon absorbs energy at a wavelength of 150. nm The total amount of energy emitted by a carbon sample is 2.23 x 105 J. Calculate the number of carbon atoms present in the sample, assuming that each atom
Source Image: opengeology.org
Download Image
PDF) Review of Active and Passive Daylighting Technologies for Sustainable Building
5 Optical Mineralogy – Mineralogy Question: Carbon absorbs energy at a wavelength of 150 nm. The total amount of energy emitted by a specific carbon sample is 1.98×105 J. Calculate the mass of the carbon sample assuming that each carbon atom emits one photon. Show transcribed image text. There are 3 steps to solve this one.
A state-of-the-art review on coir fiber-reinforced biocomposites – RSC Advances (RSC Publishing) DOI:10.1039/D1RA00231G ⏩SOLVED:Carbon absorbs energy at a wavelength of 150 . nm . The… | Numerade Calculate the energy and wavelength of a photon of light with a frequency of 6.165 x 10^14 Hz. Calculate the energy of a photon of wavelength 0.154nm; Calculate the energy of a photon having a wavelength of 5.42×10-6 m. Calculate the energy per photon of a wavelength of 6.0 x 10-10 m. Calculate the energy per photon of a wavelength of 3.0 x 10-3 m.