Draw an arrow pushing mechanism for the acid catalyzed dehydration of the following alcohol, make sure to draw both potential mechanisms. Assume no rearrangement for the first two product mechanisms. Which of these two would likely be the major product? If there was a rearrangement, draw the expected major product. Answer. 6.
Kinetic Analysis of the Hydrodeoxygenation of Aliphatic Volatilized Lignin Molecules on Bulk MoO3: Elucidating the Formation of Alkenes and Alkanes | ACS Catalysis
Q 1. Two Isomeric alkenes A and B having molecular formula, C5H 9Cl on adding H 2, A gives optically inactive compound while B gives a chiral compound. The two isomer are. View Solution. Q 2. The olefin which on ozonolysis gives CH 3CH 2CH O and CH 3CH O is: View Solution. Q 3.
Source Image: coursehero.com
Download Image
Answer and Explanation: The major product for the dehydration of 2-pentanol is a strong acid, such as phosphoric or sulphuric acid. The acid serves as a catalyst when heated with 2-pentanol under very high temperature, a process called dehydration, that eliminates water molecules and produces the alkenes, 1-pentene and 2-pentene.
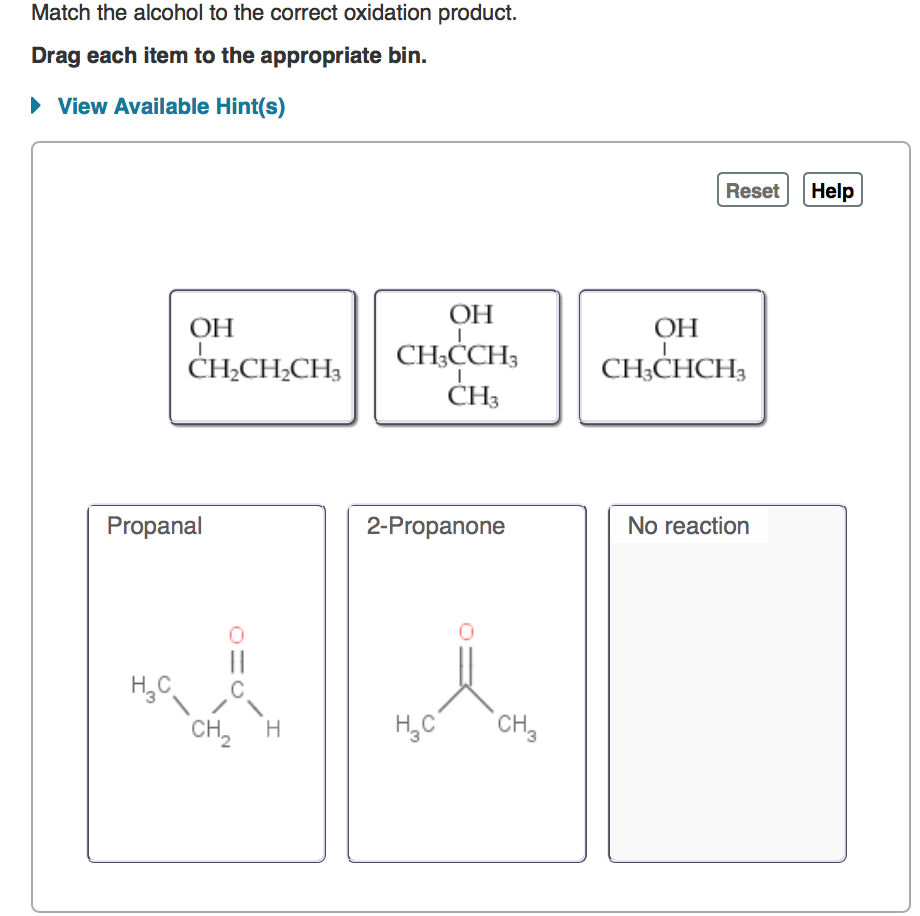
Source Image: chegg.com
Download Image
Solved Dehydration of 2-methyl-2-pentanol forms one major | Chegg.com
Draw the major product for the dehydration of 2-pentanol. | Quizlet Related questions with answers Name the aldehyde displayed below. Draw the condensed structural formula for the alcohol formed when each of the following is reduced by hydrogen in the presence of a nickel catalyst: butyraldehyde

Source Image: learnpick.in
Download Image
Draw The Major Product For The Dehydration Of 2-Pentanol.
Draw the major product for the dehydration of 2-pentanol. | Quizlet Related questions with answers Name the aldehyde displayed below. Draw the condensed structural formula for the alcohol formed when each of the following is reduced by hydrogen in the presence of a nickel catalyst: butyraldehyde
Mar 7, 2023question No one rated this answer yet — why not be the first? 😎 AshishSV The major product for the dehydration of 2-pentanol is 2-pentene. This reaction occurs through the elimination of water from the alcohol molecule. Here are the steps for the dehydration of 2-pentanol: 1. The first step is the formation of a protonated alcohol.
Alcohol,ethers – Notes – LearnPick India
Question: Part A Draw the major product for the dehydration of 2-pentanol Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars.
Structural Formula for 3-Methyl-2-pentanol (3-Methylpentan-2-ol) – YouTube

Source Image: m.youtube.com
Download Image
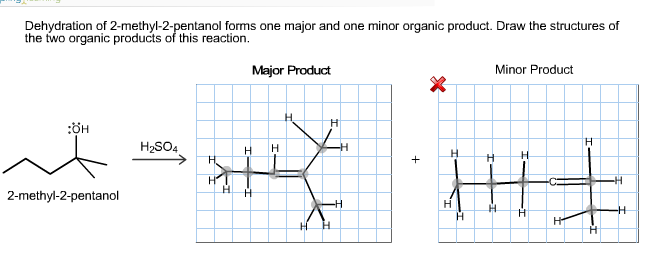
Dehydration of 2-methyl-2-pentanol forms one major and one m | Quizlet
Question: Part A Draw the major product for the dehydration of 2-pentanol Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars.

Source Image: quizlet.com
Download Image
Kinetic Analysis of the Hydrodeoxygenation of Aliphatic Volatilized Lignin Molecules on Bulk MoO3: Elucidating the Formation of Alkenes and Alkanes | ACS Catalysis
Draw an arrow pushing mechanism for the acid catalyzed dehydration of the following alcohol, make sure to draw both potential mechanisms. Assume no rearrangement for the first two product mechanisms. Which of these two would likely be the major product? If there was a rearrangement, draw the expected major product. Answer. 6.

Source Image: pubs.acs.org
Download Image
Solved Dehydration of 2-methyl-2-pentanol forms one major | Chegg.com
Answer and Explanation: The major product for the dehydration of 2-pentanol is a strong acid, such as phosphoric or sulphuric acid. The acid serves as a catalyst when heated with 2-pentanol under very high temperature, a process called dehydration, that eliminates water molecules and produces the alkenes, 1-pentene and 2-pentene.
Source Image: chegg.com
Download Image
ALCOHOLS AND ETHERS
Step 1: Write the structural formula for 2-pentanol. CH3CH2CH(OH)CH2CH3 Step 2/4 Step 2: Identify the leaving group. In this case, the leaving group is water (H2O). Step 3/4 Step 3: Determine the mechanism for the reaction. The dehydration of 2-pentanol involves an E1 mechanism, which means that the reaction proceeds through a carbocation
Source Image: yumpu.com
Download Image
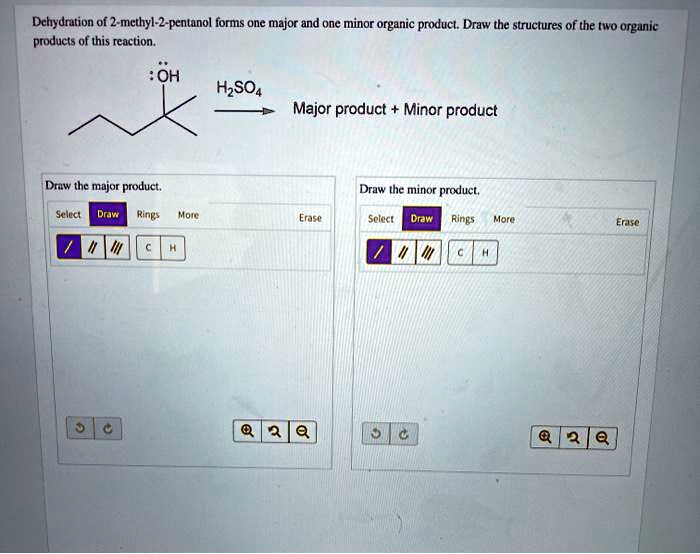
SOLVED: Dehydration of 2-methyl-2-pentanol forms one major and one minor organic product. Draw the structures of the two organic products of this reaction. OH H2SO4 Major product Minor product Draw the major
Draw the major product for the dehydration of 2-pentanol. | Quizlet Related questions with answers Name the aldehyde displayed below. Draw the condensed structural formula for the alcohol formed when each of the following is reduced by hydrogen in the presence of a nickel catalyst: butyraldehyde
Source Image: numerade.com
Download Image
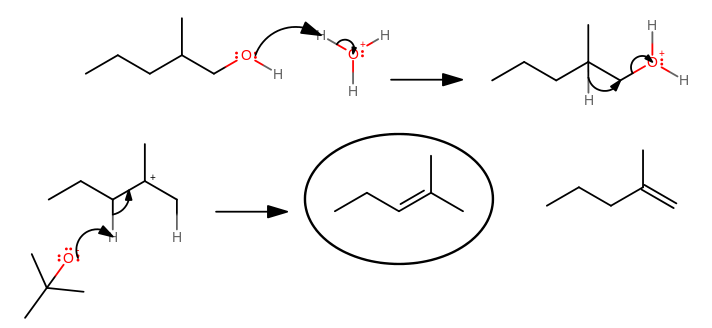
Write complete mechanism of dehydration of 2-methyl-1-pentanol? | Socratic
Mar 7, 2023question No one rated this answer yet — why not be the first? 😎 AshishSV The major product for the dehydration of 2-pentanol is 2-pentene. This reaction occurs through the elimination of water from the alcohol molecule. Here are the steps for the dehydration of 2-pentanol: 1. The first step is the formation of a protonated alcohol.
Source Image: socratic.org
Download Image
Dehydration of 2-methyl-2-pentanol forms one major and one m | Quizlet
Write complete mechanism of dehydration of 2-methyl-1-pentanol? | Socratic
Q 1. Two Isomeric alkenes A and B having molecular formula, C5H 9Cl on adding H 2, A gives optically inactive compound while B gives a chiral compound. The two isomer are. View Solution. Q 2. The olefin which on ozonolysis gives CH 3CH 2CH O and CH 3CH O is: View Solution. Q 3.
Solved Dehydration of 2-methyl-2-pentanol forms one major | Chegg.com SOLVED: Dehydration of 2-methyl-2-pentanol forms one major and one minor organic product. Draw the structures of the two organic products of this reaction. OH H2SO4 Major product Minor product Draw the major
Step 1: Write the structural formula for 2-pentanol. CH3CH2CH(OH)CH2CH3 Step 2/4 Step 2: Identify the leaving group. In this case, the leaving group is water (H2O). Step 3/4 Step 3: Determine the mechanism for the reaction. The dehydration of 2-pentanol involves an E1 mechanism, which means that the reaction proceeds through a carbocation