A compound’s molar solubility in water can be calculated from its Kₛₚ value at 25°C. To do so, first prepare an ICE (Initial, Change, and Equilibrium) table showing the equilibrium concentrations of the ions in terms of x, the molar solubility of the compound.Then, plug these expressions into the solubility-product expression for the compound and solve for the value of x.
Today is Friday (!), May 26th, ppt download
The question demands the solubility of potassium bromide at 23 degrees Celcius. As we know that the solubility of potassium bromide at 30 degrees Celcius is 70.7 and at 20 degrees Celsius it is 65.3 This question can be solved by using Lagrange Interpolation Formula which is:

Source Image: chegg.com
Download Image
The first step in the preparation of magnesium metal is the precipitation of Mg (OH) 2 from sea water by the addition of lime, Ca (OH) 2, a readily available inexpensive source of OH – ion: Mg (OH) 2 ( s) ⇌ Mg 2+ ( a q) + 2OH − ( a q) K sp = 8.9 × 10 − 12. The concentration of Mg 2+ ( aq) in sea water is 0.0537 M.
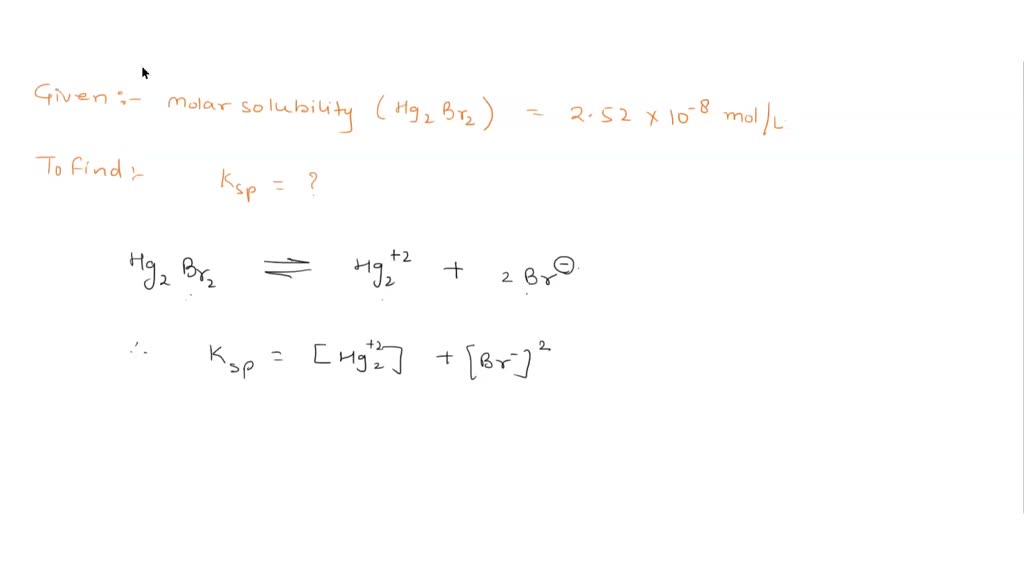
Source Image: numerade.com
Download Image
SOLVED: The enthalpy of solution for the dissolving of KBr sample was determined following Part C of the Experimental Procedure in this experiment. Complete the following table for Trial (See Report Sheet)
As stated in Section 7.9, the solvent is the substance that is reported as a 100.-gram, or 100.-milliliter, quantity in the denominator of a solubility limit. Since the chemical formula for water, H 2 O, is associated with the 100.-gram quantities in the denominators of the solubilities in Table 7.9.1, water, H 2 O, is the solvent in this

Source Image: study.com
Download Image
Calculate The Solubility Of Potassium Bromide At 23 C
As stated in Section 7.9, the solvent is the substance that is reported as a 100.-gram, or 100.-milliliter, quantity in the denominator of a solubility limit. Since the chemical formula for water, H 2 O, is associated with the 100.-gram quantities in the denominators of the solubilities in Table 7.9.1, water, H 2 O, is the solvent in this
Oct 24, 2023Solubility and Common ion Effect. In section 17.1.3 solubility was introduced as an example of the common ion effect, and this problem was explained using ICE table and Le Chatelier’s Principle.. What is the solubility of Calcium phosphate in a 0.100M sodium phosphate solution? This is the same problem as above except that there is a common ion as the soluble sodium phosphate introduces
Identifying the Qualitative Effect of Changes in pH on the Solubility of a Salt | Chemistry | Study.com
Solubility Calculator. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Enter Substance Formula (e.g. NaCl) Find Solubility. Or determine the solubility using the solubility rules or lookup table below. Finding the solubility of a substance is useful for determining net ionic equations.
sectB-answers

Source Image: yumpu.com
Download Image
SOLVED: The solubility of PbBr2 (molar mass = 367 g/mol) is 0.427 g per 100.0 mL of solution at 25°C. Determine the value of Ksp for this strong electrolyte. PbBr2(s) ⇌ Pb2+(aq) +
Solubility Calculator. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Enter Substance Formula (e.g. NaCl) Find Solubility. Or determine the solubility using the solubility rules or lookup table below. Finding the solubility of a substance is useful for determining net ionic equations.
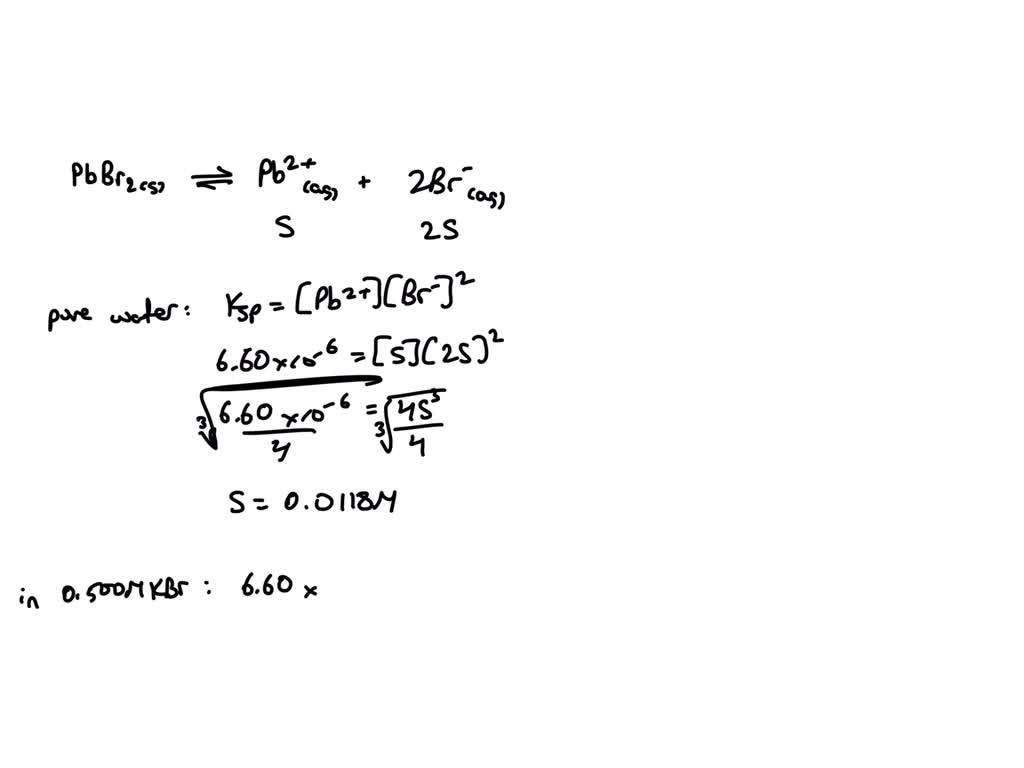
Source Image: numerade.com
Download Image
Today is Friday (!), May 26th, ppt download
The first step in the preparation of magnesium metal is the precipitation of Mg (OH) 2 from sea water by the addition of lime, Ca (OH) 2, a readily available inexpensive source of OH – ion: Mg (OH) 2 ( s) ⇌ Mg 2+ ( a q) + 2OH − ( a q) K sp = 8.9 × 10 − 12. The concentration of Mg 2+ ( aq) in sea water is 0.0537 M.

Source Image: slideplayer.com
Download Image
SOLVED: The enthalpy of solution for the dissolving of KBr sample was determined following Part C of the Experimental Procedure in this experiment. Complete the following table for Trial (See Report Sheet)
A compound’s molar solubility in water can be calculated from its Kₛₚ value at 25°C. To do so, first prepare an ICE (Initial, Change, and Equilibrium) table showing the equilibrium concentrations of the ions in terms of x, the molar solubility of the compound.Then, plug these expressions into the solubility-product expression for the compound and solve for the value of x.
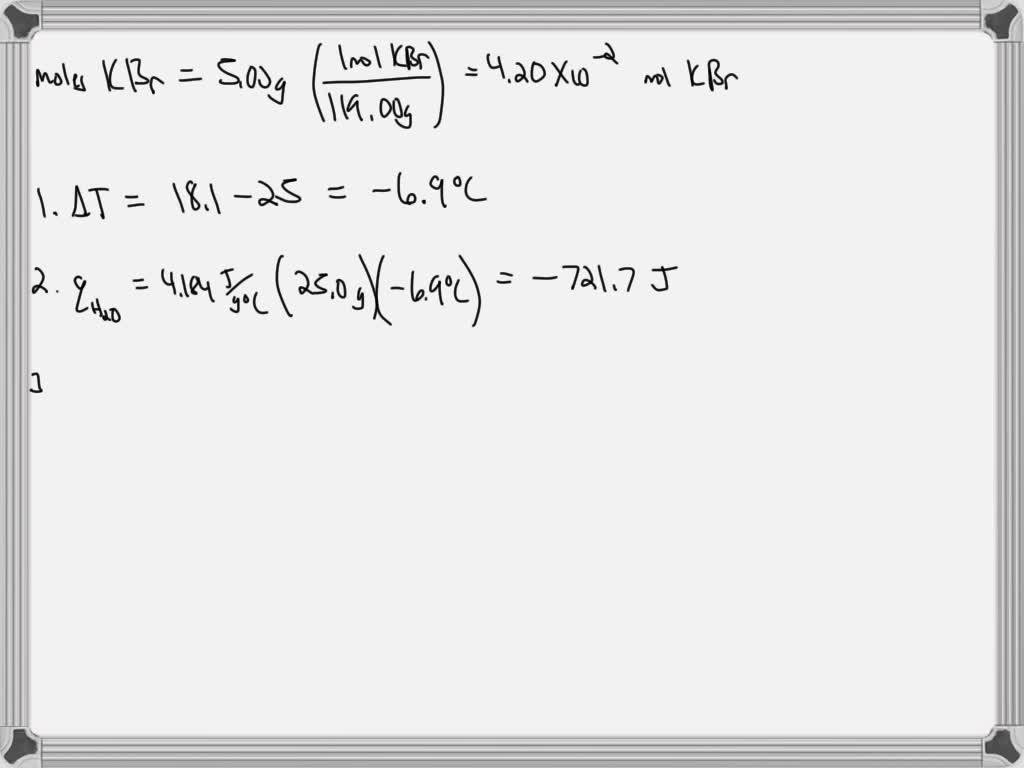
Source Image: numerade.com
Download Image
Solubility of Potassium Nitrate – 1140 Words | Report Example
Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Need a deep-dive on the concept behind this application? Look no further.
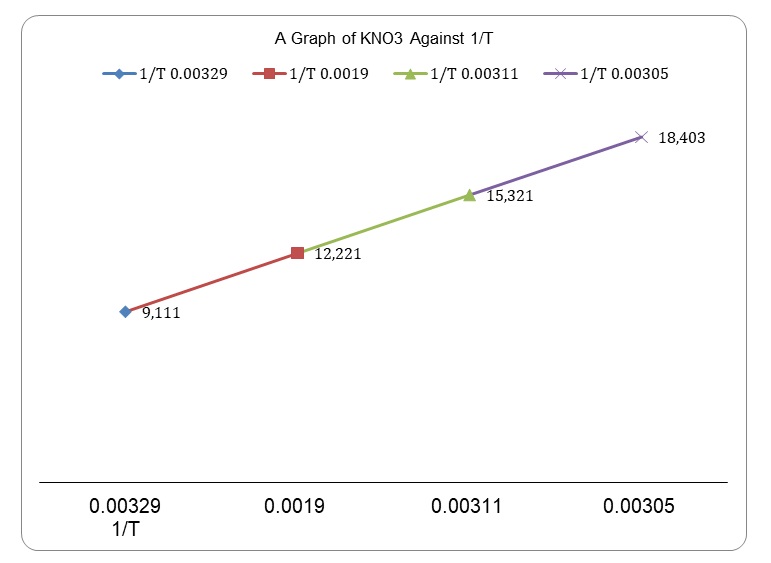
Source Image: ivypanda.com
Download Image
The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 800C.
As stated in Section 7.9, the solvent is the substance that is reported as a 100.-gram, or 100.-milliliter, quantity in the denominator of a solubility limit. Since the chemical formula for water, H 2 O, is associated with the 100.-gram quantities in the denominators of the solubilities in Table 7.9.1, water, H 2 O, is the solvent in this
 Download Image
Download ImageSolved Calculate the solubility of potassium bromide at | Chegg.com
Oct 24, 2023Solubility and Common ion Effect. In section 17.1.3 solubility was introduced as an example of the common ion effect, and this problem was explained using ICE table and Le Chatelier’s Principle.. What is the solubility of Calcium phosphate in a 0.100M sodium phosphate solution? This is the same problem as above except that there is a common ion as the soluble sodium phosphate introduces

Source Image: chegg.com
Download Image
SOLVED: The solubility of PbBr2 (molar mass = 367 g/mol) is 0.427 g per 100.0 mL of solution at 25°C. Determine the value of Ksp for this strong electrolyte. PbBr2(s) ⇌ Pb2+(aq) +
Solved Calculate the solubility of potassium bromide at | Chegg.com
The question demands the solubility of potassium bromide at 23 degrees Celcius. As we know that the solubility of potassium bromide at 30 degrees Celcius is 70.7 and at 20 degrees Celsius it is 65.3 This question can be solved by using Lagrange Interpolation Formula which is:
SOLVED: The enthalpy of solution for the dissolving of KBr sample was determined following Part C of the Experimental Procedure in this experiment. Complete the following table for Trial (See Report Sheet) The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 800C.
Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Need a deep-dive on the concept behind this application? Look no further.